Abstract
INTRODUCTION
In patients with AML who are eligible for intensive therapy, the goal of treatment is the achievement of complete response followed by consolidation chemotherapy (in favorable risk disease) or hematopoietic stem cell transplantation (in intermediate or adverse risk disease). Patients who do not attain this initial goal lack effective therapeutic options. Extensive experience with chimeric antigen receptor (CAR) T cells in B-ALL has shown that CART cells can deliver potent and durable antigen-specific leukemia control, and that targeting a single antigen (CD19 for B-ALL) is associated with antigen-negative relapse. In this context, we sought to expand the existing preclinical CART armamentarium in AML by developing FLT3-specific CART cells and comparing them to our existing gold standard CD123-specific CART cells. Since activating mutations in FLT3 occur commonly in AML, we reasoned that this molecule would serve as an "Achilles heel" in AML immunotherapy.
METHODS
Novel fully humanized anti-human FLT3 receptor single chain variable fragments (scFV) were fused to CD28 and CD137 (41BB) costimulatory molecules and the CD3zeta signaling domain and cloned into a lentiviral expression vector. Based on recently published data, we tested linker lengths ranging from 5 to 20 amino acids between the light and heavy chains of the CAR. We used a FLT3-ITD mutated AML cell line (MOLM14) expressing luciferase for in vitro function studies including an exhaustion assay. For in vivo function studies, we engrafted MOLM14 expressing luciferase into NSG mice and treated with CART-FLT3 or untransduced T cells (negative control).
RESULTS
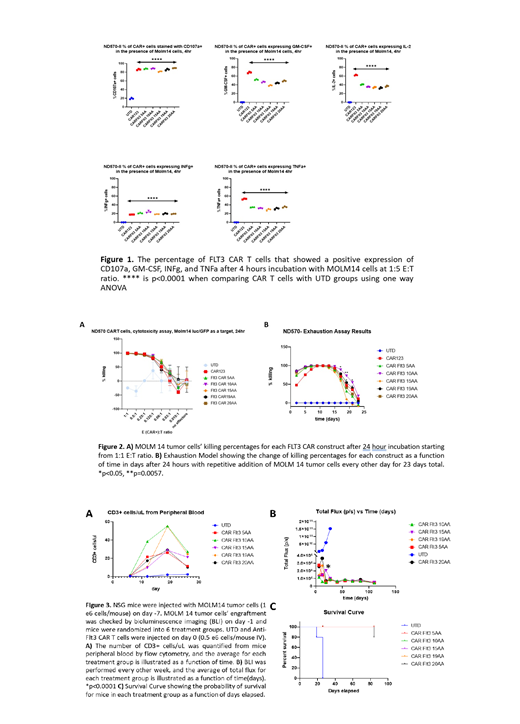
All FLT3 and CD123-specific CART cells degranulated and produced the effector cytokines IL-2, INFg, TNF and GM-CSF in an antigen-specific manner, with some variability between the different linker lengths and with some superiority of the CAR123 likely resulting from the higher expression of CD123 compared with FLT3 in this model (p < 0.0001, one way ANOVA) (Figure 1). Short-term killing assays (24 hours) revealed that all CART cells killed MOLM14 with equivalent efficiency at low effector:target ratios (Figure 2A). Since short-term killing assays likely do not replicate the physiological situation in vivo wherein CART cells encounter cancer cells repeatedly over many days, we next developed an in vitro exhaustion assay. We incubated MOLM14 cells with CAR T cells at 1:10 E:T ratio and added MOLM 14 tumor cells along with fresh media every other day. Killing was quantified every 48 hours. Interestingly, all CAR constructs showed equivalently efficient cytotoxicity from days 5-15. However, after day 15 there was progressive dysfunction and loss of cytotoxic activity. This exhaustion "stress test" revealed some superiority of the FLT3 CAR 10AA construct (p = 0.042 on day 17, two way ANOVA) (Figure 2B).
NOD/SCID gamma chain KO (NSG) mice were then engrafted with 1x10 6 luciferized MOLM14 cells and treated with 0.5x10 6 CAR T cells 7 days later, randomized to treatment groups based on tumor burden. CAR T cells expansion was monitored in peripheral blood by flow cytometry. (Fig 3A). Serial BLI revealed prompt and durable leukemia remissions and survival (Figure 3B,C).
CONCLUSIONS
We have developed CART-FLT3 for AML using novel human anti-FLT3 targeting domains and demonstrated preclinical efficacy similar to that of CART-123 in an AML model with substantially lower expression of FLT3 compared to CD123 (data not shown). Since inhibition of FLT3 leads to upregulation of surface FLT3 expression, future experiments will explore combinatorial FLT3 inhibition with CART-FLT3. If successful, these experiments will provide a strong rationale for a combination clinical trial in AML where leukemia control by small molecules is followed by a coup-de-grace delivered by CART cells.
Sleiman: Hemogenyx Pharmaceuticals LLC: Research Funding. Shestova: Hemogenyx Pharmaceuticals LLC: Research Funding. Santiago: Hemogenyx Pharmaceuticals LLC: Research Funding. Shrestha: Hemogenyx Pharmaceuticals LLC: Current Employment. Liang: Hemogenyx Pharmaceuticals LLC: Current Employment. Ben Jehuda: Hemogenyx Pharmaceuticals LLC: Current Employment. Sandler: Hemogenyx Pharmaceuticals LLC: Current Employment, Current equity holder in publicly-traded company. Gill: Novartis: Other: licensed intellectual property, Research Funding; Interius Biotherapeutics: Current holder of stock options in a privately-held company, Research Funding; Carisma Therapeutics: Current holder of stock options in a privately-held company, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal